Rapid Antigen Testing Segment Expected to Hold Significant Share of Rapid Test Kits Market During 2022–2030
According to our latest study on "Rapid Test Kits Market Forecast to 2030 – Global Analysis – Type, Product, Technology, Application, and End User" the market was valued at US$ 17.72 billion in 2022 and is expected to reach US$ 26.63 billion by 2030, recording a CAGR of 5.2% during 2022–2030. Factors such as the rising prevalence of genetic and infectious diseases, and strategic initiatives by manufacturers bolster the rapid test kits market size. However, uncertainties related to the accuracy of results impede the rapid test kits market growth.
Operations of multiplex rapid antigen tests are based on multicolored nanoparticles and cross-reactive antibodies, and these test kits can be developed at low costs. When various respiratory pathogens are circulating simultaneously in a host body, rapid multiplex diagnostic testing helps healthcare providers supervise their patients with greater effectiveness. These tests can help avoid misdiagnosis that is possible due to overlapping clinical symptoms. By eliminating diagnostic uncertainty, they help healthcare providers make apt decisions about the commencement and discontinuation of antimicrobial therapies. Unlike single specimen antigen test kits, multiplex rapid antigen test kits can detect many respiratory viruses from a single specimen, without compromising the quality of results. On behalf of the UK-Rapid Test Consortium (UK-RTC), the UK Government has awarded a contract to Abingdon Health for the procurement of the AbC-19 rapid antibody tests. The contract follows an independent evaluation of this rapid antibody test, commissioned by the UK Government. Therefore, innovations such as multiplex rapid antigen tests are expected to emerge as new rapid test kits market trends in the coming years.
Published Report - Rapid Test Kits Market Size and Forecasts (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Type (Rapid Antigen Testing, Rapid Antibody Testing, and Others), Product (Over-the-Counter Rapid Testing Kit and Professional Rapid Testing Kit), Technology (Lateral Flow Assay, Solid Phase, Agglutination, Immunospot Assay, and Cellular Component-Based), Application (Blood Glucose Testing, Infectious Disease Testing, Pregnancy and Fertility, Cardiometabolic Testing, and Others), End User (Hospital and Clinics, Home Care, Diagnostics Centers, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)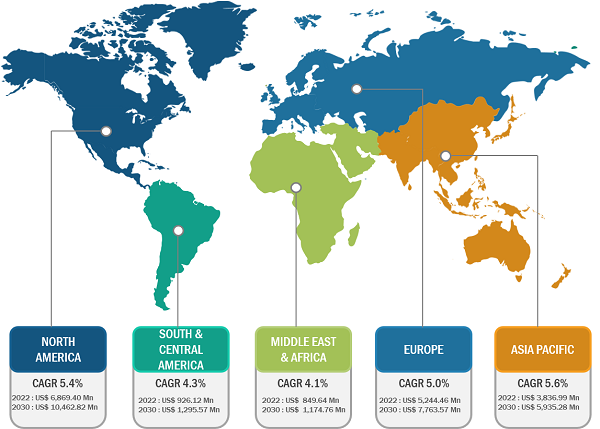
Rapid Test Kits Market Growth, Analysis, Size, Share by 2030
Download Free Sample
Market Segmentation:
The rapid test kits market analysis has been carried out by considering the following segments: product, application, and end user.
Rapid Test Kits Market, by Product:
Based on product, the market is bifurcated into over-the-counter rapid testing kits and professional rapid testing kits. The over-the-counter rapid testing kit segment held a larger rapid test kits market share in 2022. It is anticipated to register a higher CAGR of 5.7% during the forecast period. A large number of rapid home tests delivering fast and precise results are now available in the market. The use of over-the-counter rapid testing kits allows users to easily know the infection status at an early stage, which can help prevent the spread of viral infection to others. Presently, all FDA-approved COVID-19 antigen tests that may be performed at home are permitted for serial testing. Mylab CoviSelf, Genabio COVID-19 Rapid Self-Test Kit, i-Can One Step Pregnancy Test Kit and OraQuick In-Home HIV test are a few of the examples of over-the-counter rapid testing kits available in the market.
Rapid Test Kits Market, by Application:
By application, the market is segmented into blood glucose testing, infectious disease testing, pregnancy and fertility, cardiometabolic testing, and others. The blood glucose testing segment held the largest rapid test kits market share in 2022. It is anticipated to register the highest CAGR of 5.7% during the forecast period. The amount of blood glucose, which is the primary source of energy in the human body, is determined via a blood glucose test. Insulin is a naturally occurring hormone that facilitates the uptake of glucose into cells from blood. Many people with diabetes find that blood sugar testing aids in managing their disease as well as preventing complications. Many methods can be employed to measure blood sugar. A continuous glucose monitor (CGM) uses a small sensor to detect blood sugar levels 24/7. Blood glucose monitoring facilitates the identification of trends in blood glucose (sugar) fluctuations resulting from dietary changes, physical activity, medication interactions, and pathological processes such as diabetes mellitus.
Rapid Test Kits Market, by End User:
The rapid test kits market, by end user, is categorized into hospitals and clinics, diagnostics centers, home care, and others. The hospital and clinics segment held the largest market share in 2022. Hospitals and clinics provide healthcare services such as diagnostics and disease treatment with the help of modernized equipment. The increasing number of hospital admissions as well as a subsequently high prevalence of chronic diseases contribute to the growth of the rapid test kits market for the hospital segment. Emerging nations are witnessing high demand for advanced hospital settings to manage a huge patient pool and address public health concerns. Thus, a rise in the number of hospitals and clinics is anticipated to boost the adoption of diagnostic tests, including rapid test kits, in the coming years.
The home care segment is anticipated to register the highest CAGR of 5.7% during 2022–2030. This segment is further divided into point-of-care testing and patient self-testing. Emerging countries are witnessing a gradual shift in patient preference from hospital settings to homecare settings. This factor is prominently expected to boost the growth of the rapid test kits market for the homecare segment. Diabetes care devices allow patients suffering from diabetes to monitor and manage their disease at home. The home care market majorly constitutes patients having type 1 diabetes. The prevalence of type 1 diabetes is associated with the increased demand for diabetes care devices. Moreover, HIV self-test (or rapid self-test) and COVID-19 antibody tests can be used at home or in a private location.
Rapid Test Kits Market, by Geography:
The geographic scope of the rapid test kits market report entails North America (US, Canada, and Mexico), Europe (Spain, UK, Germany, France, Italy, and Rest of Europe), Asia Pacific (South Korea, China, India, Japan, Australia, and Rest of Asia Pacific), Middle East & Africa (South Africa, Saudi Arabia, UAE, and Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and Rest of South & Central America). The rapid test kits market growth in North America is fueled by increasing demand for innovative products from bioanalytical instrument manufacturers.
The US accounted for a significant share of the North American market as the country invests notably in advanced research methodologies, which favors the rapid test kits market progress. There was a rapid increase in the use of at-home tests after the outbreak of the SARS-CoV-2 Delta- and Omicron variants. According to the CDC, from late 2020 till May 2022, more than 70 million test kits were shipped across the US. Moreover, the country is investing significantly in the establishment and operations of small biotechnology startups to provide support innovation through new analytical technologies such as real-time PCR.
Apart from factors driving the market, the rapid test kits market report emphasizes prominent players operating in the market; these include F. Hoffmann-La Roche Ltd, Becton Dickinson and Co, ARKRAY Inc, Sysmex Partec GmbH, Fujirebio Europe NV, bioMerieux SA, Cepheid, Meril Life Sciences Pvt Ltd, QIAGEN NV, OraSure Technologies Inc, Guangzhou Wondfo Biotech Co Ltd, Denka Co Ltd, Abbott Laboratories, Trinity Biotech Plc, SD Biosensor Inc, Bio-Rad Laboratories Inc, Hologic Inc, DiaSorin SpA, Premier Medical Corp Pvt Ltd, and Beckman Coulter Inc.
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com